How Dewpoint is Affected by Pressure
Dewpoint is a measurement of water vapor concentration. Water vapor, or more simply “moisture”, can be a major contaminant in process gases ranging from compressed air, to purified cylinder gases, to hydrocarbon gases, to natural gas, and many others. Moisture content can affect the gases’ quality and the detrimental effects of poor-quality process gases can manifest in various ways including:
- Corrosion of equipment and piping systems
- Damage to manufacturing equipment
- Micro-organism growth
- Effect on manufactured product quality
It is extremely important to realize that dewpoint is a function of pressure. In other words, depending on the pressure of the system being measured, the sample gas can have an infinite number of dewpoints, all dependent on the pressure of the system. The reason for this dependency on pressure is that dewpoint is an absolute indication (i.e. measurement) of water vapor pressure. Water vapor pressure is one of potentially many partial pressures that makeup a gas mixture. Each component of a gas mixture exerts its own partial pressure. The sum of all the component partial pressures equals the total pressure of the system. This last statement is essentially Dalton’s Law of partial pressures. Therefore, if the pressure of a system is altered, the partial pressure of all the components in the gas mixture will all change by the same ratio.
Example: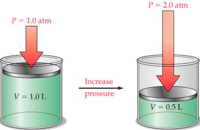
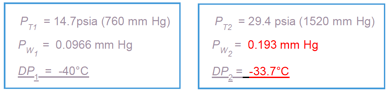
Explanation:
The piston depicted in the graphic is mechanically forced downward resulting in a doubling of the system pressure, from 14.7 psia to 29.4 psia. In accordance with Dalton’s Law, all the partial pressures of the individual components also double. That raises the water vapor pressure from 0.0966 mm HG to 0.193 mm Hg. Because dewpoint is an absolute measurement of water vapor pressure, the change in water vapor pressure results in a change in the dewpoint from -40oC to -33.7oC.
Because of the dependency of pressure, dewpoint measurements are commonly made at one of two convenient pressure levels: process pressure or atmospheric pressure. For a given water vapor content, the higher the process pressure, the wetter the dewpoint. The factors normally used to assist with this decision are usually either that the dewpoint at line/process pressure is required, so the dewpoint measurement is accurate for the process line conditions, or the gas is allowed to expand (normally over a needle valve) to atmospheric pressure, ensuring the dewpoint readings that are always relative to atmospheric pressure.
The chart below indicates “equivalent dewpoint” at various process pressure: